Bresslergroup is now Delve!
Created in 1970


Up & running (A)
Existing signals show a regular activitySocial networks
2,306 5,690 17,042Activities
Technologies
Entity types
Location
1216 Arch St 7th floor, Philadelphia, PA 19107, USA
Philadelphia
United States of America
Employees
Scale: 201-500, 51-200
Estimated: 518
Engaged catalyst
14
0 0Added in Motherbase
5 years, 8 months ago
Value proposition
Bresslergroup is now Delve. Visit us at Delve.com.
Bresslergroup is now Delve. Visit us at Delve.com.
Engineering Analysis & Optimization, Design & Innovation Strategy, Design Research, Medical Product Design, Digital-Physical Design, Technology-Based Innovation, Product Brand Language, Industrial & Interaction Design, Mechanical & Electrical Engineering, Internet of Things, Software Engineering, and Rugged & Portable Products
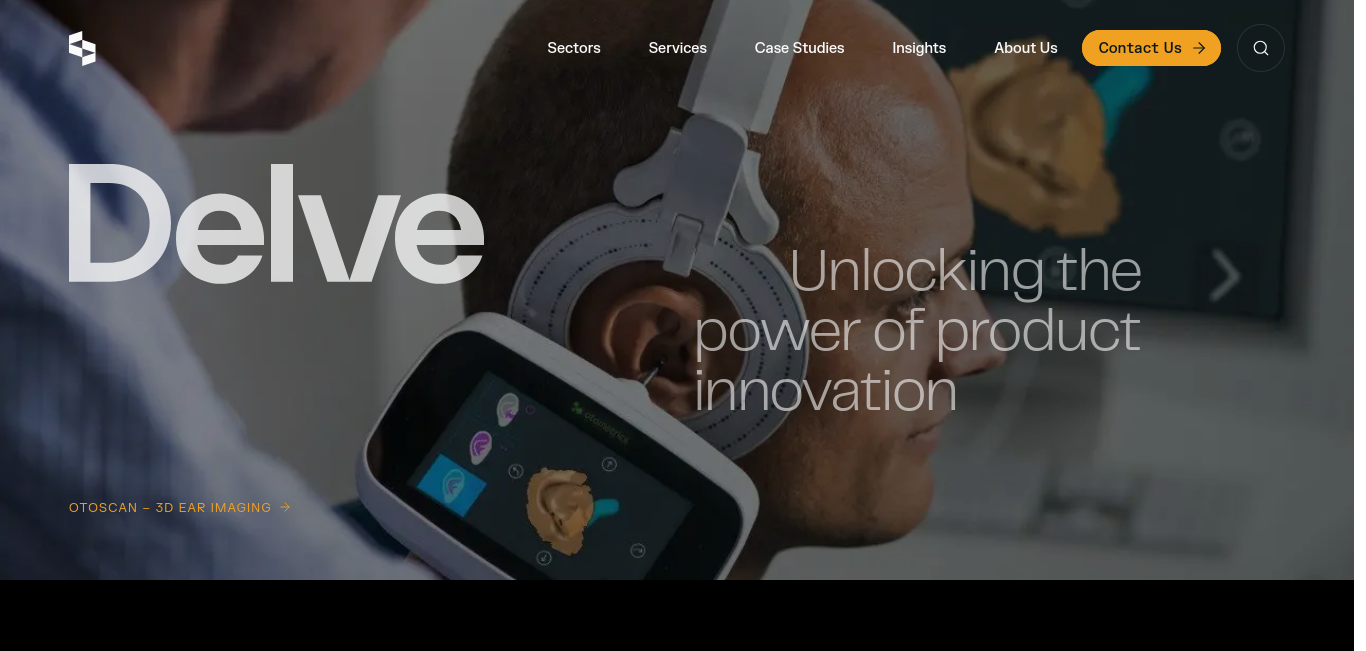
Delve Product Design and Development | Bring Bold Ideas to… | Delve
Product engineers, designers, and user researchers at Delve have delivered 10,000+ projects for the medical, industrial, and consumer industries.
https://www.delve.com/
| Catalyst | Type | Tweets | Articles | |
|---|---|---|---|---|
 Cisco IT services, Software Development | Cisco IT services, Software Development | Other 20 Dec 2017 | | |
 InterDigital, Inc. Consumer Electronics, Telecommunications | InterDigital, Inc. Consumer Electronics, Telecommunications | Not capitalistic Partnership Event 28 Mar 2017 | | |
 UN Africa Renewal International development, International Affairs | UN Africa Renewal International development, International Affairs | Other 31 Mar 2020 | | |
 Bosch Manifacturing tools, Software Development | Bosch Manifacturing tools, Software Development | Other 27 Jul 2018 | | |
 Consumer Technology Association Trade show, Computers and Electronics Manufacturing | Consumer Technology Association Trade show, Computers and Electronics Manufacturing | Other 13 Feb 2020 | | |
 Intel Corporation IT services, Semiconductors, Semiconductor Manufacturing | Intel Corporation IT services, Semiconductors, Semiconductor Manufacturing | Other 10 Jun 2019 | | |
 Nasdaq Finance, Financial Services | Nasdaq Finance, Financial Services | Other 11 Dec 2017 | | |
 L'Oréal Cosmetics, Personal Care Product Manufacturing | L'Oréal Cosmetics, Personal Care Product Manufacturing | Not capitalistic Partnership Not event 10 Jan 2018 | | |
 IBM IT services, IT Services and IT Consulting | IBM IT services, IT Services and IT Consulting | Other 6 Jan 2020 | | |
 Adobe IT services, Software Development | Adobe IT services, Software Development | Not capitalistic Not partnership Event 14 Mar 2018 | | |
BEEP. BEEP. BEEP. Alarms should save lives, not add to the noise.
But in many hospitals, medical device alarms go off thousands of times a day. Over time, clinicians start tuning them out.
Then, when a real emergency happens, a critical alarm gets missed—and the patient pays the price.
The fix? Smarter alarm design.
At Delve, this is just one example of how we help medtech teams build safer, more intuitive devices—by avoiding the design traps that lead to overload, confusion, and risk.
Swipe through the slideshow for seven critical design considerations that reduce alarm fatigue and improve safety.
🔹 Want to go deeper? The full article breaks down best practices for effective alarm design.
🔹 Need a quick-reference guide? Download our free poster to keep your team aligned.
Links in the comments.
#HumanFactors #MedicalDevices #healthcare
Are you heading to Toronto for the International Symposium on Human Factors and Ergonomics in Health Care? Amazing. Us, too. The leaders of Delve’s Human Factors team are presenting and attending, and we’d love to connect with you.
There are two ways to get into contact with us at HFES:
- Curt Irwin, Senior Director of Human Factors Engineering, will discuss Smarter Medical Device Alarm Design on April 1, 4:45-6:15 pm, in the Frontenac Foyer
- Email connect@delve.com with your availability and we’ll respond in < 24 hours
We’re excited for thoughtful conversations, fresh perspectives—and maybe even a double-double or two. Let’s connect.
#HumanFactors #MedicalDevices #HFES2025
New clients mean new opportunities. Join us at Delve in shaping the future of product design and engineering.
For medtech, consumer health, and energy/sustainability equipment companies seeking to innovate beyond their core, Delve is the product innovation firm that both conceives and ruthlessly derisks creative solutions to seemingly intractable innovation challenges. We’re looking for top talent across software engineering, project management, business development, software development, and more to join our growing team.
Swipe through the slideshow to see some of our open roles. If you're looking for your next challenge or know someone who is, now’s a great time to make a move—or help someone else make theirs.
Apply today. Or tag someone who should see this. Links to open roles in the comments.
#Hiring #ProductDesign #ProductEngineering
🚨 Medical device development teams can’t afford to cut corners. Not now.
With the recent FDA staffing reductions and ongoing restructuring, pre-submission meetings could get harder to schedule, and reviews may take longer. That’s a problem if your submission isn’t airtight.
Too many teams waste time fixing avoidable mistakes—gaps in risk management, sloppy human factors testing, disorganized DHFs. And the FDA is paying attention:
🔹 Fewer than 10% of human factors submissions pass on the first try.
🔹 Common failures? Weak use-risk analyses and usability tests that don’t reflect real-world conditions.
🔹 The result? Costly delays.
How to avoid this:
✔️ Risk management is a mindset, not a box to check. Build it into your process early, not as a last-minute fix.
✔️ Human factors are under the microscope. Test like your submission depends on it—because it does.
✔️ Your Design History File (DHF) is your safety net. Make it clear, complete, and unbreakable.
FDA bottlenecks are coming. If your device isn’t rock-solid, you could be waiting longer than expected to get to market.
We help medtech teams navigate these challenges—balancing speed with compliance.
Our latest article breaks down why regulatory uncertainty is growing—and what medtech teams can do to stay ahead. Link in comments.
How is your team preparing for potential delays?
#productdevelopment #FDA #medtech
We’re thrilled to share that Colin McCloskey has joined Delve as Director of Software Engineering, adding additional horsepower to our digital offering, which grew more than 25% last year. This growth aligns with the broader trend, as demand for products that cross the physical and digital divide, Delve’s core expertise, is projected to grow at an annualized rate of 11.2% from 2024 to 2030.
Colin M. joins Delve after nearly eight years at Arcweb Technologies, where he served as Head of Engineering, overseeing technology strategy, leading high-performing teams, and driving innovation across healthcare, insurance, and financial sectors. This included the development of an open-source platform for digital health and clinical applications which enhanced data management efficiency and accelerated product development cycles. Prior to Arcweb, Colin spent time at Gusto, CNET, and as a founding and early member of startup companies with a focus on platform, infrastructure, and social network development.
As our CEO Patrick Frend put it: "Too many companies treat digital and physical product development as separate worlds. That’s a mistake. The best products today live at the intersection of both. With Colin joining our team, we’re continuing to sharpen our competitive edge and helping clients innovate faster and smarter, whether they’re launching a connected medical device or building a cutting-edge digital health platform."
Welcome to the team, Colin. We’re excited to have you on board as we continue to drive the future of digital-physical product development.
#softwareengineering #productdevelopment #engineering